Introduction
In a historic medical milestone, the U.S. Food and Drug Administration (FDA) has approved a groundbreaking therapy for sickle cell disease (SCD) utilizing CRISPR-Cas9 gene-editing technology. This approval marks a significant advancement in the treatment of a disease that affects millions of people worldwide, predominantly those of African descent. Sickle cell disease is a genetic disorder that causes red blood cells to assume a rigid, sickle shape, leading to various complications such as pain, infections, and strokes.
Understanding Sickle Cell Disease
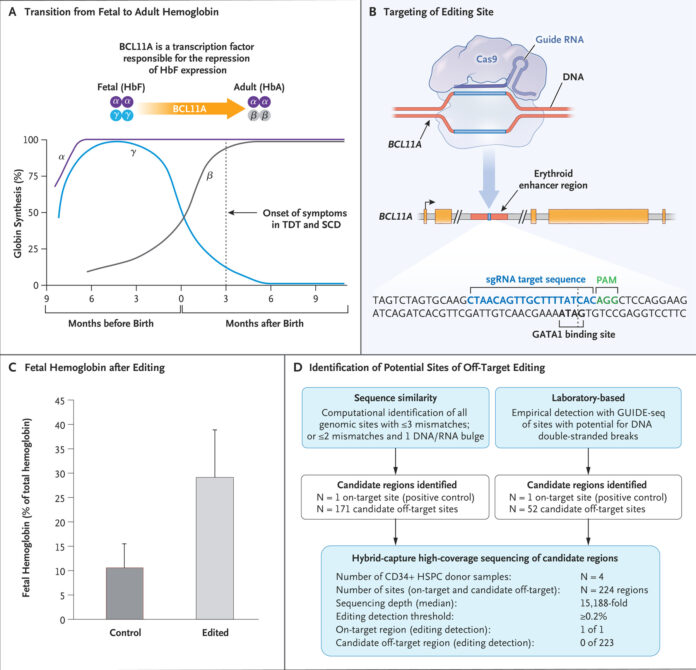
Sickle cell disease is caused by a mutation in the HBB gene, which encodes the beta-globin subunit of hemoglobin. This mutation leads to the production of abnormal hemoglobin known as hemoglobin S (HbS). When deoxygenated, HbS molecules polymerize, causing red blood cells to deform into a sickle shape. These sickle-shaped cells are prone to getting stuck in blood vessels, obstructing blood flow and leading to painful vaso-occlusive crises, chronic hemolysis, and organ damage.
The CRISPR-Cas9 Technology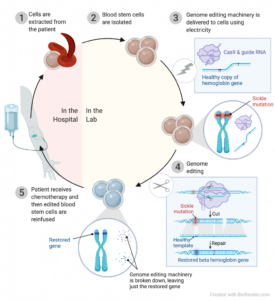
CRISPR-Cas9, an acronym for Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9, is a revolutionary gene-editing tool that allows for precise, targeted changes to the DNA of living organisms. Discovered in bacteria, CRISPR-Cas9 acts as a defense mechanism against viral infections by cutting the DNA of invading viruses. Scientists have harnessed this system to edit genes in a wide range of organisms, including humans.
Development of the CRISPR Therapy for Sickle Cell Disease
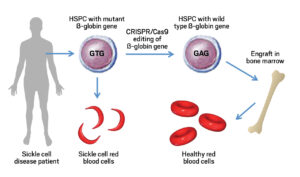
The development of the CRISPR-based therapy for sickle cell disease has been spearheaded by researchers at various institutions, including CRISPR Therapeutics and Vertex Pharmaceuticals. The therapy, known as CTX001, involves extracting hematopoietic stem cells from the patient, using CRISPR-Cas9 to edit the cells to produce fetal hemoglobin (HbF) instead of hemoglobin S, and then reintroducing these edited cells into the patient’s body.
Fetal hemoglobin, which is naturally present in fetuses and newborns, can effectively prevent the sickling of red blood cells. By reactivating the production of fetal hemoglobin in patients with sickle cell disease, the therapy aims to reduce the frequency and severity of vaso-occlusive crises and other complications.
Clinical Trials and Approval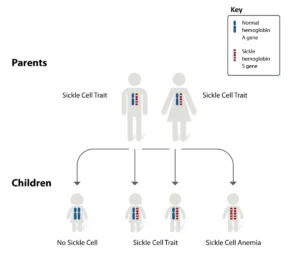
Clinical trials for CTX001 have shown promising results. In a landmark study published in the New England Journal of Medicine, researchers reported that patients treated with CTX001 experienced a significant reduction in vaso-occlusive crises and improved overall health. One patient, Victoria Gray, became the first person with sickle cell disease to be treated with the CRISPR-based therapy, and her positive response has been a beacon of hope for many.
The FDA’s approval of CTX001 is based on the robust data from these clinical trials, which demonstrated the safety and efficacy of the therapy. The approval process involved rigorous evaluation of the trial results, including long-term follow-up data to ensure the durability of the treatment’s effects.
Implications for Patients
The approval of CTX001 offers a new lease on life for patients with sickle cell disease. Traditional treatments, such as hydroxyurea and blood transfusions, primarily manage symptoms but do not address the underlying genetic cause of the disease. CTX001, on the other hand, targets the root cause by editing the patient’s own stem cells to produce healthy red blood cells.
This therapy has the potential to significantly improve the quality of life for patients, reducing hospitalizations and the need for chronic pain management. Moreover, the success of CTX001 opens the door for further advancements in gene-editing therapies for other genetic disorders.
Ethical Considerations and Future Directions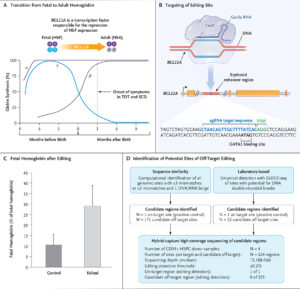
The use of CRISPR-Cas9 in human therapies raises several ethical considerations. The potential for off-target effects, where unintended genes are edited, poses risks that need to be carefully managed. Additionally, the long-term effects of gene editing are still not fully understood, necessitating ongoing research and monitoring.
Despite these challenges, the approval of CTX001 represents a major step forward in the field of genetic medicine. It exemplifies the potential of gene-editing technologies to transform the treatment of genetic diseases. Future research may expand the applications of CRISPR-Cas9 to other conditions, such as thalassemia, cystic fibrosis, and muscular dystrophy.
Conclusion
The FDA’s approval of the CRISPR-based therapy CTX001 for sickle cell disease is a monumental achievement in medical science. This innovative therapy offers hope to millions of patients worldwide, providing a treatment that addresses the genetic root cause of the disease. As research and technology continue to advance, the potential for CRISPR-Cas9 to revolutionize the treatment of genetic disorders is immense, heralding a new era in precision medicine.